Workstream &
Work packages
New approaches to diagnosing respiratory illnesses
Human breath is an attractive sample for diagnostics due to its non-invasive collection method. Traditional breath-based diagnostics focus on volatile organic compounds (VOCs), which lack specificity and cannot detect antimicrobial resistance (AMR). A recent VOC-based test has been authorized for emergency use but still requires confirmation by molecular testing. Direct molecular pathogen detection in breath offers higher specificity and the ability to detect AMR.
Swab sampling of the upper respiratory tract can be prone to sampling error, is uncomfortable for patients, and often misses lower respiratory tract pathogens.
Sputum is often difficult to obtain and transport and has suboptimal sensitivity for many respiratory pathogens; moreover, the production of sputum varies according to pathogen and individual immune response, and methods to induce sputum are resource consuming and not scalable.
Recent technological advances in molecular diagnostics have enabled ultra-sensitive detection of respiratory pathogens closer to the patient, making the sampling technique the major limiting step. Molecular testing also comes with the advantage of rapid detection of AMR, offering better tools to tackle the global AMR crisis.
Human breath has been considered a sample for diagnosing disease since ancient times. It is an attractive sample type due to ease of collection via non-invasive methods. For many years, the focus of breath-based diagnostics has been on volatile organic compounds (VOCs). VOC-based breath diagnostics fall short on specificity (typically 60-80%) and are not able to detect AMR, as their origins are usually metabolic, and can be influenced by several disease processes, conditions, and host factors. A few breath-sampling devices have been granted US FDA authorization and CE marking in the last few years but they still require in deep analysis and evaluation.
Furthermore, breath is linked to transmission of infectious microorganisms via the respiratory route, thus a breath test can conceivably detect the most infectious individuals. Aerosol transmission of respiratory infections has been largely underestimated, with the COVID-19 pandemic highlighting the limitations of traditional views of droplet and fomite transmission. There is now robust evidence supporting aerosol transmission of many respiratory infections including pandemic coronaviruses, influenza, respiratory syncytial virus (RSV), and TB. Available research tools to better our understanding of aerosols can efficiently collect XBAs and detect pathogens in over 90% of patients, but are highly technical and resource intensive (e.g., the Respiratory Aerosol Sampling Chamber [RASC] developed by consortium partners).
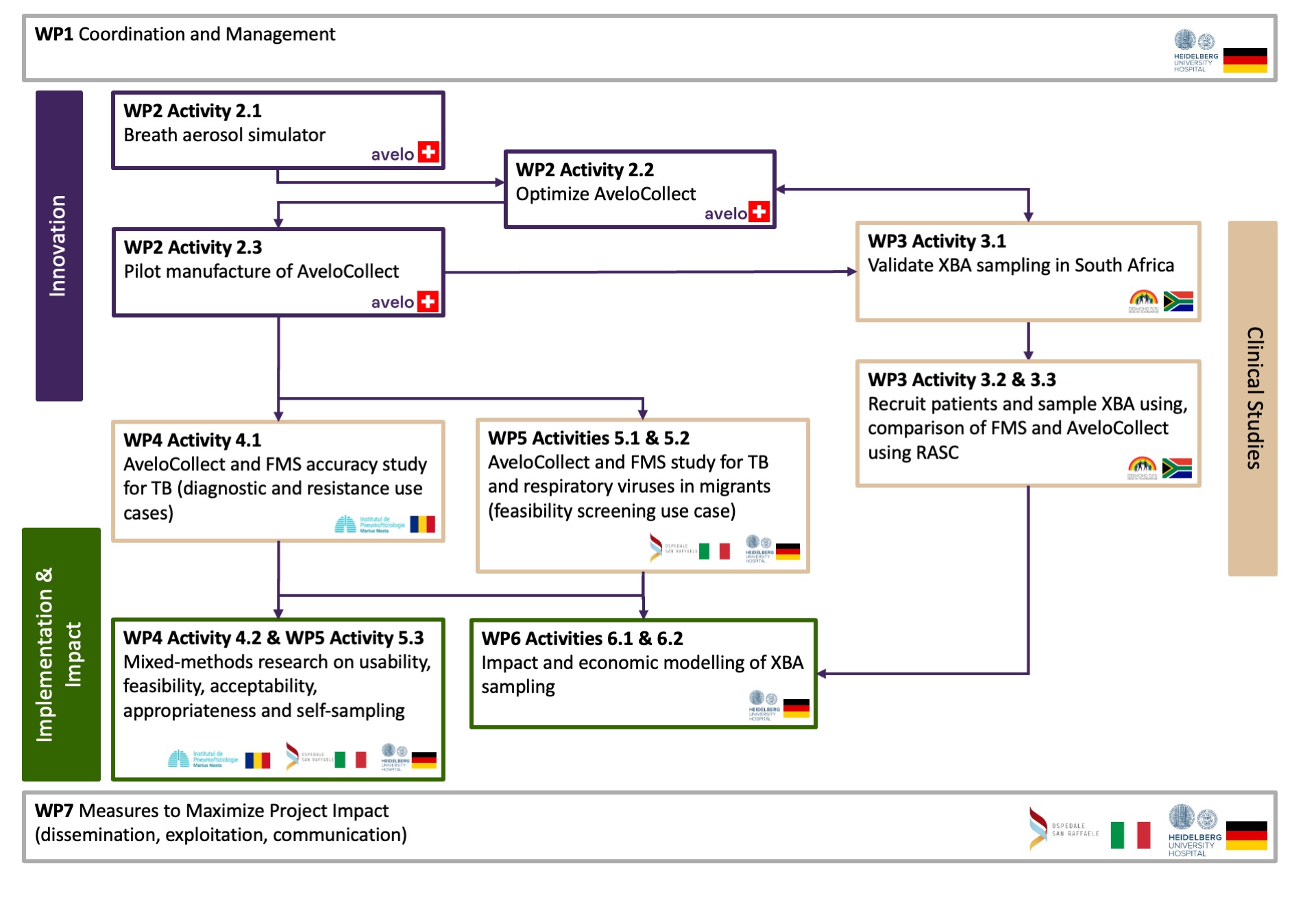
work package 1
work package 2

work package 3
Comparison of XBA sampling devices using Respiratory Aerosol Sampling Chamber (RASC).
work package 4
A diagnostic accuracy study of XBA sampling and testing using both AveloCollect and FMS will be performed in Romania, a country with a high-burden of DR-TB, aiming to screen 600 and recruit 300 patients with TB symptoms (100 Xpert Ultra positive, and 200 Xpert Ultra negative patients). In parallel to the diagnostic accuracy study, we will apply mixed-methods research grounded in CFIR to compare their perceived acceptability, usability and perceived appropriateness to ultimately inform diagnostic and care management decisions among participants, experienced senior HCWs, and other important implementation partners.
work package 5
A coordinated protocol for Tuberculosis (TB) and respiratory viruses screening using XBA in a selected sample of temporary migrant, first-arrival centres in Italy and Germany will be developed in collaboration with local and national health and immigration authorities. Molecular testing will be done Mtb, SARS-CoV-2, influenza and Respiratory Syncytial Virus (RSV) at local laboratories.
Feasibility, acceptability, and determinants of implementation and scale-up in the screening use case will be assessed.
A coordinated protocol for screening for TB and respiratory viruses using XBA in a selected sample of temporary migrant, first-arrival centres in Italy and Germany will be developed in collaboration with local and national health and immigration authorities. We will screen 2270 migrants for symptoms and enrolment, aiming for XBA samples using both AveloCollect and FMS devices to be collected in 568 symptomatic and 568 asymptomatic individuals, divided equally between each country, from eligible consenting participants. Molecular testing will be done with Xpert Ultra for Mtb and Xpert Xpress for SARS-CoV-2, influenza and RSV (multiplexed) at local laboratories.
Feasibility, acceptability, and determinants of implementation and scale-up in the screening use case will be assessed.
work package 6
The full economic cost of XBA testing developed will be evaluated and validated under WPs 2, 3, and 4, respectively, if it were to be implemented in both high-income EU countries, and low-income, high TB burden countries.
A compartmental model of TB transmission will be developed in two key epidemiological settings (low and high TB burden), with the XBA testing being used for screening.
work package 7
Reach out to society to show impact and benefits of project activities; dissemination and networking, making knowledge and results available to scientists, health care professionals and others that can build upon and learn from the findings; policy dialogue and optimal exploitation of results, to facilitate their translation into new policies.
A professional corporate identity will be provided with the collaboration of all partners. The public project website will inform about all aspects of the project, including the team behind the research, recent project achievements, and upcoming events. Short videos will be distributed; press releases will be centrally coordinated by UKHD and will be distributed on key dates. Social media will be a crucial tool for reaching out to the scientific community and the lay public.
Community engagement webinars will be held and, in the end, exploitation activities will be planned in order to make concrete use of project findings both in the field of scientific research and in public health policies and clinical practice.





